The Food Safety and Standards Authority of India (FSSAI) has announced key amendments to its food labeling regulations, aimed at providing consumers with clearer and more prominent nutritional information, particularly regarding added sugar, saturated fat, and sodium. The changes also mandate a specific logo for milk and milk products, and stricter front-of-pack declarations for coffee-chicory mixtures. This draft notification was published on February 20, 2025.
Highlighting Unhealthy Components
The draft amendment proposes that the per-serve percentage (%) contribution to the Recommended Dietary Allowance (RDA) for added sugar, saturated fat, and sodium be displayed in bold letters with an increased font size. This aims to draw consumers’ attention to these potentially harmful components, enabling them to make informed and healthier choices.
Specific Logo for Milk and Milk Products
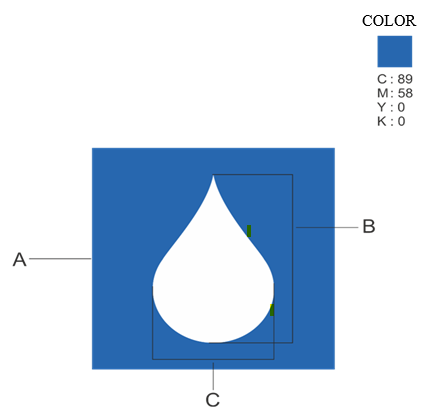
To ensure clear identification of milk and milk products, the FSSAI is seeking to introduce a mandatory logo. The logo, featuring a stylized milk drop within a square, will be required on all milk and milk products, including composite milk products, as defined under the Food Safety and Standards (Food Products Standards and Food Additives) Regulations, 2011.
The size of the logo will vary depending on the area of the principal display panel, with specific dimensions provided for different sizes of packaging.
Stricter Front-of-Pack Declarations for Coffee-Chicory Mixtures
The regulations for coffee-chicory mixtures are proposed to be tightened. Declarations regarding the percentage of coffee and chicory, previously required, will now be displayed prominently on the front of the package, in capital letters within a rectangular box on the principal display panel. This ensures that consumers can easily identify the composition of the product.
Stakeholder Feedback
Objections or suggestions on this draft amendment, if any, may be addressed to the Chief Executive Officer, Food Safety and Standards Authority of India, FDA Bhawan, Kotla Road, New Delhi- 110002 or may be sent by e-mail
at regulation@fssai.gov.in. The feedback shall be furnished in the specified format. The deadline for furnishing the feedback is April 20, 2025.
You may also be interested in:
Natamycin Recognized As Food Additives For Fermented Milks And Dairy Based Desserts
Government Initiatives to Boost Milk Production in India
FSSAI Introduces New Draft Regulations for Primary Milk Producers

