The Central Drugs Standard Control Organization (CDSCO) has launched a new, streamlined online Export No Objection Certificate (NOC) system on the SUGAM portal, aimed at enhancing “ease of doing business” for pharmaceutical exporters. This public notice was issued on March 7, 2025.
This initiative simplifies the process of obtaining export NOCs for unapproved and approved new drugs, reducing compliance burdens and facilitating smoother export procedures.
Key Features of the New System
1-Year Validity NOCs
To reduce compliance burdens, CDSCO will now issue 1-year validity NOCs for eligible drugs, subject to prescribed conditions.
Revised SUGAM Checklist and Guidance Document:
The SUGAM checklist has been updated, and a comprehensive guidance document has been released to assist applicants.
Online Application Process
All export NOC applications must be submitted through the SUGAM portal, along with the prescribed checklist of documents.
The process involves a one-time registration at the zonal office, followed by consignment release procedures at the port office.
Applicants must complete an Integrated Registration Form (IRF) once, which is valid for one year.
The zonal office will verify the IRF, and a 1-year validity NOC will be issued within 7 working days.
After obtaining the valid export NOC, applicants must submit dynamic consignment details online for verification by the port office.
Detailed reconciliation data, test/batch release certificates, and purchase order/export invoice/shipping bill details are required for both registration and consignment release.
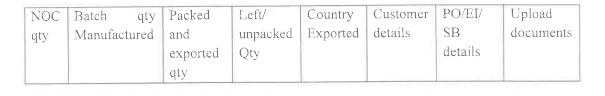
Discontinuation of Quantity/PO Specific NOCs
Quantity-specific and Purchase Order (PO)-specific NOCs are being discontinued, except for Narcotic Drugs and Psychotropic Substances (NDPS) and drugs.
Un-exported quantities can be used for subsequent export orders within 60% of the residual shelf life. If the shelf life is below 60% the material must be destroyed in the presence of the State Licensing Authority.
The system ensures a 7-working-day timeline for NOC issuance, with two-level processing for timely disposal.
For NDPS and drugs, the existing quantity-specific and PO-specific NOC system will remain in place.
This initiative is expected to significantly benefit pharmaceutical exporters by simplifying and expediting the export NOC process, thereby boosting India’s pharmaceutical exports.

